How to Take
FEMLYV
Place a FEMLYV tablet on your tongue1
Allow it to disintegrate, followed by water1
What to Do if You Miss Tablets1
FEMLYV may not be as effective if you miss any green tablets, especially if you miss the first few or the last few green tablets in a pack
- Take the tablet as soon as you remember. Take the next tablet at your regular time. This means you may take 2 tablets in 1 day.
- You do not need to use a backup birth control method if you have sex.
OC, oral contraceptive; ODT, orally disintegrating tablet.
Before You Start Taking
Your FEMLYV Tablets1
1
Decide what time of day
you want to take your tablet1
It is important to take FEMLYV tablets in the order
directed on the package at the same time every day.
FEMLYV can be taken with or without meals
2
Look at your tablet pack—
it has 28 tablets1
The FEMLYV pack has 24 "active" green tablets
(with hormones) to be taken for 24 days, followed
by 4 "reminder" white tablets (without hormones)
to be taken for the next four days
3
Also look for1:
a) Where on the pack to start taking tablets
b) In what order to take the tablets (follow
the arrows as shown in the picture)
c) The week numbers as shown
4
Be sure you have ready
at all times1:
a) Another kind of birth control (such as a
condom and spermicide) to use as a backup
in case you miss tablets, and
b) An extra, full tablet pack
How to Take
FEMLYV
Before You Start
Taking Your
FEMLYV Tablets1
1
Decide what time of
day you want to
take your tablet1
It is important to take
FEMLYV tablets in the order
directed on the package at
the same time every day.
FEMLYV can be taken with
or without meals
2
Look at your
tablet pack—it
has 28 tablets1
The FEMLYV pack has
24 "active" green tablets
(with hormones) to be taken
for 24 days, followed by
4 "reminder" white tablets
(without hormones) to
be taken for the next
four days
3
Also look for1:
a) Where on the pack to
start taking tablets
b) In what order to take
the tablets (follow the
arrows as shown in
the picture)
c) The week numbers as
shown
4
Be sure you
have ready
at all times1:
a) Another kind of birth
control (such as a
condom and spermicide)
to use as a backup
in case you miss tablets,
and
b) An extra, full tablet pack
How to Take
FEMLYV
Place a FEMLYV tablet on your tongue1
Allow it to disintegrate, followed by water1

Take one tablet at the same time
every day until the pack is empty.1

Do not skip tablets even if you are
spotting or bleeding between monthly
periods or feel sick to your stomach
(nausea). Do not skip tablets even if
you do not have sex very often.1
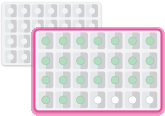
When you finish a pack of tablets,
start the next pack on the day after
your last white tablet. Do not wait
any days between packs.1
What to Do if
You Miss Tablets1
FEMLYV may not be as effective if you
miss any green tablets, especially if
you miss the first few or the last
few green tablets in a pack
- Take the tablet as soon as you remember. Take the next tablet at your regular time. This means you may take 2 tablets in 1 day.
- You do not need to use a backup birth control method if you have sex.
OC, oral contraceptive; ODT, orally disintegrating tablet.

Take one tablet
at the same time
every day until the
pack is empty.1

Do not skip tablets
even if you are
spotting or bleeding
between monthly
periods or feel sick
to your stomach
(nausea). Do not
skip tablets even
if you do not have
sex very often.1

When you finish
a pack of tablets,
start the next pack
on the day after
your last white
tablet. Do not
wait any days
between packs.1
What to Do
if You Miss
Tablets1
FEMLYV may not be
as effective if you
miss any green
tablets, especially if
you miss the first
few or the last
few green tablets
in a pack
- Take the tablet as soon as you remember. Take the next tablet at your regular time. This means you may take 2 tablets in 1 day.
- You do not need to use a backup birth control method if you have sex.
OC, oral contraceptive; ODT, orally disintegrating tablet.
IMPORTANT SAFETY INFORMATION
WARNING TO WOMEN WHO SMOKE
Do not use FEMLYV if you smoke cigarettes and are over 35 years old. Smoking increases your risk of serious cardiovascular side effects (heart and blood vessel problems) from birth control pills, including death from heart attack, blood clots or stroke. This risk increases with age and the number of cigarettes you smoke.
What is FEMLYV?
FEMLYV is a birth control pill. It contains two female hormones, an estrogen called ethinyl estradiol, and a progestin called norethindrone acetate.
Who should not take FEMLYV?
Your healthcare provider will not give you FEMLYV if you have ever had blood clots or are at a high risk for stroke, heart attack or other heart problems or abnormalities, breast cancer, liver disease, including liver tumors, take any Hepatitis C drug combination containing ombitasvir, paritaprevir, ritonavir, with or without dasabuvir, which may increase levels of the liver enzyme “alanine aminotransferase” (ALT) in the blood, or have any abnormal bleeding from the vagina. Tell your healthcare provider if you have ever had any of the above conditions (your healthcare provider may recommend another method of birth control). Do not take birth control pills if you smoke and are over 35 years old, are or think you are pregnant, or have any abnormal bleeding from the vagina.
What else should I know about FEMLYV?
You should stop FEMLYV at least 4 weeks before you have surgery and not restart it until at least 2 weeks after the surgery, due to an increased risk of blood clots. If you are breastfeeding, consider another birth control method until you are ready to stop breastfeeding. Birth control pills that contain estrogen, like FEMLYV, may decrease the amount of milk you make. A small amount of the pill's hormones passes into breast milk.
What are the most serious risks of taking FEMLYV?
Like pregnancy, birth control pills increase the risk of serious blood clots, especially in women who have other risk factors, such as smoking, obesity, or age greater than 35. This increased risk is highest when you first start taking birth control pills and when you restart the same or different birth control pills after not using them for a month or more. It is possible to die from a problem caused by a blood clot, such as a heart attack or a stroke.
Call your healthcare provider right away if you have leg pain that does not go away, sudden shortness of breath, sudden blindness (partial or complete), severe pain or pressure in your chest, sudden, severe headache unlike your usual headaches, weakness or numbness in an arm or leg, or trouble speaking, or yellowing of the skin or eyeballs.
What are the common side effects of birth control pills?
The most common side effects of birth control pills are spotting or bleeding between menstrual periods, nausea, breast tenderness, and headache. These side effects are usually mild and usually disappear with time. The most common adverse reactions in clinical trials (greater than or equal to 2%) were: headache, vaginal candidiasis, nausea, menstrual cramps, breast tenderness, bacterial vaginitis, abnormal cervical smear, acne, mood swings, and weight gain.
These are not all the possible side effects of FEMLYV. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Please see the full Prescribing Information, including BOXED WARNING, and Patient Information.
References: 1. FEMLYV [package insert]. East Hanover, NJ: Millicent Pharma Limited; 2024. 2. FDA Approves Femlyv (norethindrone acetate and ethinyl estradiol) Orally Disintegrating Birth Control Pill. Drugs.com. July 2024. Accessed August 5, 2024. www.drugs.com/newdrugs/fda-approves-femlyv-norethindrone-acetate-ethinyl-estradiol-orally-disintegrating-birth-control-pill-6330.html 3. Data on File. Millicent Pharma Limited. 4. Nakajima ST, Archer DF, Ellman H. Efficacy and safety of a new 24-day oral contraceptive regimen of norethindrone acetate 1 mg/ethinyl estradiol 20 µg (Loestrin® 24 Fe). Contraception. 2007;75(1):16-22. 5. Fumero A, Marrero RJ, Peñate W, Bethencourt JM, Barreiro P. Adherence to oral contraception in young women: beliefs, locus of control, and psychological reactance. Int J Environ Res Public Health. 2021;18(21):11308. 6. Christin-Maitre S. History of oral contraceptive drugs and their use worldwide. Best Pract Res Clin Endocrinol Metab. 2013;27(1):3-12. 7. Chewable birth control pill unveiled. CBS News. December 8, 2006. Accessed August 5, 2024. www.cbsnews.com/news/chewable-birth-control-pill-unveiled/ 8. Allergan announces launch of TAYTULLA™ (norethindrone acetate and ethinyl estradiol capsules and ferrous fumarate capsules) 1 mg/20 mcg, the first and only oral contraceptive softgel capsule. PR Newswire. November 10, 2016. Accessed August 5, 2024. https://www.prnewswire.com/news-releases/allergan-announces-launch-of-taytulla-norethindrone-acetate-and-ethinyl-estradiol-capsules-and-ferrous-fumarate-capsules-1-mg20-mcg-the-first-and-only-oral-contraceptive-softgel-capsule-300360428.html
IMPORTANT SAFETY INFORMATION
WARNING TO WOMEN WHO SMOKE
Do not use FEMLYV if you smoke cigarettes and are over 35 years old. Smoking increases your risk of serious cardiovascular side effects (heart and blood vessel problems) from birth control pills, including death from heart attack, blood clots or stroke. This risk increases with age and the number of cigarettes you smoke.
What is FEMLYV?
FEMLYV is a birth control pill. It contains two female hormones, an estrogen called ethinyl estradiol, and a progestin called norethindrone acetate.
Who should not take FEMLYV?
Your healthcare provider will not give you FEMLYV if you have ever had blood clots or are at a high risk for stroke, heart attack or other heart problems or abnormalities, breast cancer, liver disease, including liver tumors, take any Hepatitis C drug combination containing ombitasvir, paritaprevir, ritonavir, with or without dasabuvir, which may increase levels of the liver enzyme “alanine aminotransferase” (ALT) in the blood, or have any abnormal bleeding from the vagina. Tell your healthcare provider if you have ever had any of the above conditions (your healthcare provider may recommend another method of birth control). Do not take birth control pills if you smoke and are over 35 years old, are or think you are pregnant, or have any abnormal bleeding from the vagina.
What else should I know about FEMLYV?
You should stop FEMLYV at least 4 weeks before you have surgery and not restart it until at least 2 weeks after the surgery, due to an increased risk of blood clots. If you are breastfeeding, consider another birth control method until you are ready to stop breastfeeding. Birth control pills that contain estrogen, like FEMLYV, may decrease the amount of milk you make. A small amount of the pill's hormones passes into breast milk.
What are the most serious risks of taking FEMLYV?
Like pregnancy, birth control pills increase the risk of serious blood clots, especially in women who have other risk factors, such as smoking, obesity, or age greater than 35. This increased risk is highest when you first start taking birth control pills and when you restart the same or different birth control pills after not using them for a month or more. It is possible to die from a problem caused by a blood clot, such as a heart attack or a stroke.
Call your healthcare provider right away if you have leg pain that does not go away, sudden shortness of breath, sudden blindness (partial or complete), severe pain or pressure in your chest, sudden, severe headache unlike your usual headaches, weakness or numbness in an arm or leg, or trouble speaking, or yellowing of the skin or eyeballs.
What are the common side effects of birth control pills?
The most common side effects of birth control pills are spotting or bleeding between menstrual periods, nausea, breast tenderness, and headache. These side effects are usually mild and usually disappear with time. The most common adverse reactions in clinical trials (greater than or equal to 2%) were: headache, vaginal candidiasis, nausea, menstrual cramps, breast tenderness, bacterial vaginitis, abnormal cervical smear, acne, mood swings, and weight gain.
These are not all the possible side effects of FEMLYV. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Please see the full Prescribing Information, including BOXED WARNING, and Patient Information.
References: 1. FEMLYV [package insert]. East Hanover, NJ: Millicent Pharma Limited; 2024. 2. FDA Approves Femlyv (norethindrone acetate and ethinyl estradiol) Orally Disintegrating Birth Control Pill. Drugs.com. July 2024. Accessed August 5, 2024. www.drugs.com/newdrugs/fda-approves-femlyv-norethindrone-acetate-ethinyl-estradiol-orally-disintegrating-birth-control-pill-6330.html 3. Data on File. Millicent Pharma Limited. 4. Nakajima ST, Archer DF, Ellman H. Efficacy and safety of a new 24-day oral contraceptive regimen of norethindrone acetate 1 mg/ethinyl estradiol 20 µg (Loestrin® 24 Fe). Contraception. 2007;75(1):16-22. 5. Fumero A, Marrero RJ, Peñate W, Bethencourt JM, Barreiro P. Adherence to oral contraception in young women: beliefs, locus of control, and psychological reactance. Int J Environ Res Public Health. 2021;18(21):11308. 6. Christin-Maitre S. History of oral contraceptive drugs and their use worldwide. Best Pract Res Clin Endocrinol Metab. 2013;27(1):3-12. 7. Chewable birth control pill unveiled. CBS News. December 8, 2006. Accessed August 5, 2024. www.cbsnews.com/news/chewable-birth-control-pill-unveiled/ 8. Allergan announces launch of TAYTULLA™ (norethindrone acetate and ethinyl estradiol capsules and ferrous fumarate capsules) 1 mg/20 mcg, the first and only oral contraceptive softgel capsule. PR Newswire. November 10, 2016. Accessed August 5, 2024. https://www.prnewswire.com/news-releases/allergan-announces-launch-of-taytulla-norethindrone-acetate-and-ethinyl-estradiol-capsules-and-ferrous-fumarate-capsules-1-mg20-mcg-the-first-and-only-oral-contraceptive-softgel-capsule-300360428.html
IMPORTANT SAFETY INFORMATION
WARNING TO WOMEN WHO SMOKE
Do not use FEMLYV if you smoke cigarettes and are over 35 years old. Smoking increases your risk of serious cardiovascular side effects (heart and blood vessel problems) from birth control pills, including death from heart attack, blood clots or stroke. This risk increases with age and the number of cigarettes you smoke.
What is FEMLYV?
FEMLYV is a birth control pill. It contains two female hormones, an estrogen called ethinyl estradiol, and a progestin called norethindrone acetate.
Who should not take FEMLYV?
Your healthcare provider will not give you FEMLYV if you have ever had blood clots or are at a high risk for stroke, heart attack or other heart problems or abnormalities, breast cancer, liver disease, including liver tumors, take any Hepatitis C drug combination containing ombitasvir, paritaprevir, ritonavir, with or without dasabuvir, which may increase levels of the liver enzyme “alanine aminotransferase” (ALT) in the blood, or have any abnormal bleeding from the vagina. Tell your healthcare provider if you have ever had any of the above conditions (your healthcare provider may recommend another method of birth control). Do not take birth control pills if you smoke and are over 35 years old, are or think you are pregnant, or have any abnormal bleeding from the vagina.
What else should I know about FEMLYV?
You should stop FEMLYV at least 4 weeks before you have surgery and not restart it until at least 2 weeks after the surgery, due to an increased risk of blood clots. If you are breastfeeding, consider another birth control method until you are ready to stop breastfeeding. Birth control pills that contain estrogen, like FEMLYV, may decrease the amount of milk you make. A small amount of the pill's hormones passes into breast milk.
What are the most serious risks of taking FEMLYV?
Like pregnancy, birth control pills increase the risk of serious blood clots, especially in women who have other risk factors, such as smoking, obesity, or age greater than 35. This increased risk is highest when you first start taking birth control pills and when you restart the same or different birth control pills after not using them for a month or more. It is possible to die from a problem caused by a blood clot, such as a heart attack or a stroke.
Call your healthcare provider right away if you have leg pain that does not go away, sudden shortness of breath, sudden blindness (partial or complete), severe pain or pressure in your chest, sudden, severe headache unlike your usual headaches, weakness or numbness in an arm or leg, or trouble speaking, or yellowing of the skin or eyeballs.
What are the common side effects of birth control pills?
The most common side effects of birth control pills are spotting or bleeding between menstrual periods, nausea, breast tenderness, and headache. These side effects are usually mild and usually disappear with time. The most common adverse reactions in clinical trials (greater than or equal to 2%) were: headache, vaginal candidiasis, nausea, menstrual cramps, breast tenderness, bacterial vaginitis, abnormal cervical smear, acne, mood swings, and weight gain.
These are not all the possible side effects of FEMLYV. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Please see the full Prescribing Information, including BOXED WARNING, and Patient Information.
References: 1. FEMLYV [package insert]. East Hanover, NJ: Millicent Pharma Limited; 2024. 2. FDA Approves Femlyv (norethindrone acetate and ethinyl estradiol) Orally Disintegrating Birth Control Pill. Drugs.com. July 2024. Accessed August 5, 2024. www.drugs.com/newdrugs/fda-approves-femlyv-norethindrone-acetate-ethinyl-estradiol-orally-disintegrating-birth-control-pill-6330.html 3. Data on File. Millicent Pharma Limited. 4. Nakajima ST, Archer DF, Ellman H. Efficacy and safety of a new 24-day oral contraceptive regimen of norethindrone acetate 1 mg/ethinyl estradiol 20 µg (Loestrin® 24 Fe). Contraception. 2007;75(1):16-22. 5. Fumero A, Marrero RJ, Peñate W, Bethencourt JM, Barreiro P. Adherence to oral contraception in young women: beliefs, locus of control, and psychological reactance. Int J Environ Res Public Health. 2021;18(21):11308. 6. Christin-Maitre S. History of oral contraceptive drugs and their use worldwide. Best Pract Res Clin Endocrinol Metab. 2013;27(1):3-12. 7. Chewable birth control pill unveiled. CBS News. December 8, 2006. Accessed August 5, 2024. www.cbsnews.com/news/chewable-birth-control-pill-unveiled/ 8. Allergan announces launch of TAYTULLA™ (norethindrone acetate and ethinyl estradiol capsules and ferrous fumarate capsules) 1 mg/20 mcg, the first and only oral contraceptive softgel capsule. PR Newswire. November 10, 2016. Accessed August 5, 2024. https://www.prnewswire.com/news-releases/allergan-announces-launch-of-taytulla-norethindrone-acetate-and-ethinyl-estradiol-capsules-and-ferrous-fumarate-capsules-1-mg20-mcg-the-first-and-only-oral-contraceptive-softgel-capsule-300360428.html
FEMLYV™ is a trademark of Millicent Manufacturing PR, LLC.
Distributed by Millicent U.S. Inc., East Hanover, NJ 07936.
The Millicent Pharma logo is a registered trademark of Millicent Pharma Limited.
© 2025, Millicent Pharma Limited. All rights reserved. 11/2025
LYV011R1
FEMLYV™ is a trademark of Millicent Manufacturing PR, LLC.
Distributed by Millicent U.S. Inc., East Hanover, NJ 07936.
The Millicent Pharma logo is a registered trademark of Millicent Pharma Limited.
© 2025, Millicent Pharma Limited. All rights reserved. 11/2025
LYV011R1








